Scientists could be a step closer to treating drug-resistant mesial temporal lobe epilepsy (MTLE).
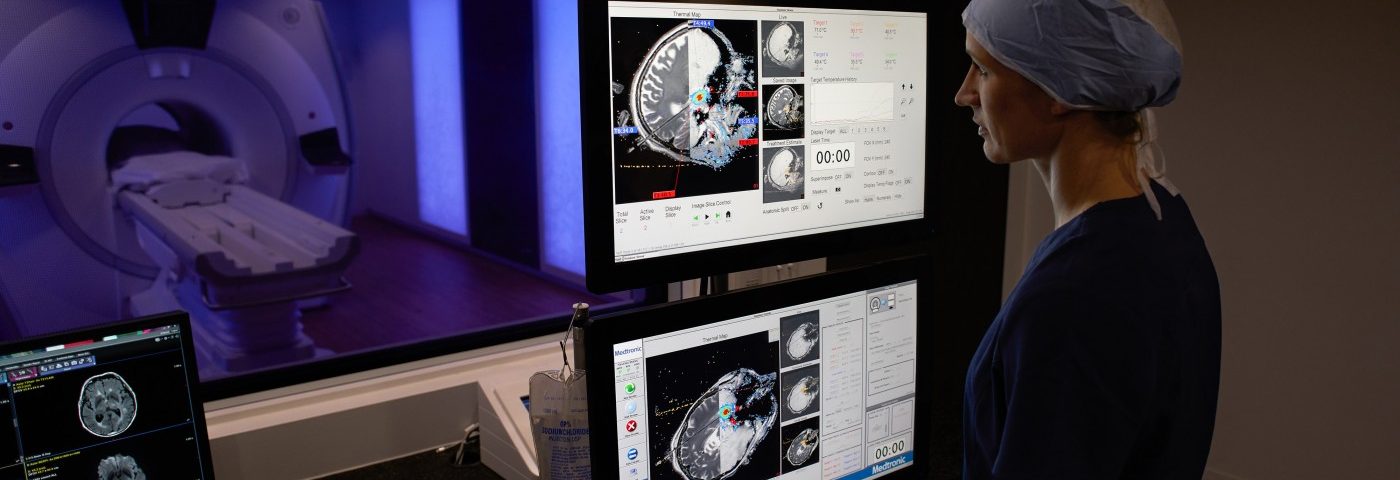
Physicians at the Mayo Clinic in Rochester, Minnesota, recently performed the first procedure testing an MRI-guided laser ablation technology called Visualase to treat MTLE. Medtronic, which developed Visualase, said it was part of a clinical trial called SLATE (Stereotactic Laser Ablation for Temporal Lobe Epilepsy).
“The SLATE study is an example of Medtronic’s commitment to conduct rigorous clinical research in partnership with physicians and providers,” Brett Wall, senior vice president and president of Medtronic’s Brain Therapies division, said in a press release. He added that SLATE “offers the opportunity to gather clinical evidence that can help us transform the way epilepsy is treated.”
Visualase uses an MRI-guided laser to precisely target and treat small areas of the brain. It is a minimally invasive procedure, but is not yet approved for the treatment of epilepsy and may now only be used for research purposes.
The SLATE trial (NCT02844465) will test the safety and efficacy of Visualase in 120 adults with MTLE. It is now recruiting participants at various sites across the United States including Emory University, Mayo Clinic, Northwell Health, Thomas Jefferson University Hospital and University of Miami Health System.
Researchers will follow participants for a year after the procedure and assess them in terms of seizure freedom, quality of life, adverse side effects and neuropsychological outcomes.
“We’re excited about this important milestone and look forward to continued recruitment in this study, which will determine the utility of the technology as a surgical treatment for patients with drug-resistant MTLE,” said Dr. Michael Sperling, a neurology professor at Philadelphia’s Thomas Jefferson University who, along with Dr. Robert Gross, was the study’s co-principal investigator.
“The data collected in this study will allow a comprehensive evaluation of the risks and benefits of the device in this new indication, ensuring that treating physicians are well-informed about its use,” said Gross, director of Emory’s Functional, Stereotactic & Epilepsy Surgery Division.
MTLE is the most common form of partial or localization-related epilepsy. About a third of MTLE patients become resistant to drug treatment and keep having seizures regardless of the type and number of antiepileptic drugs.


Are children a part of this study?
Dear Karla,
Thanks for your message. No, the technology is currently being tested in adults only.